Metabolic signaling functions of ER–mitochondria contact sites: role in metabolic diseases
- 1Department of Clinical Sciences, Lund University Diabetes Centre, Malmö, Sweden
- 2INSERM UMR-1060, CarMeN Laboratory, Lyon 1 University, INRA U1235, INSA of Lyon, Charles Merieux Lyon-Sud medical Universities, Lyon, France
- Correspondence should be addressed to J Rieusset; Email: jennifer.rieusset{at}univ-lyon1.fr
-
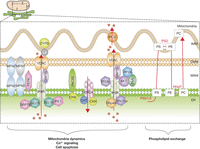
Figure 1
Schematic representation of ER–mitochondria contact sites and the established cellular signaling. Several proteins implicated in mitochondria dynamics, Ca2+ signaling, cellular apoptosis or phospholipid exchange at MAM interface are depicted. In yellow mitochondria and in green the ER, the red arrows correspond to calcium flux. ER, endoplasmic reticulum; IMM, inner mitochondrial membrane; MAM, mitochondria-associated membranes; OMM, outer mitochondrial membrane.
-
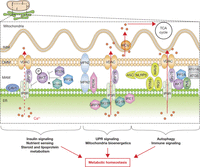
Figure 2
MAM is an important hub for several signaling pathways controlling metabolic homeostasis. The new role of MAM in insulin and nutrient signaling and in steroid and lipoprotein metabolism, as well as their role in unfolded protein response (UPR) signaling, mitochondria bioenergetics, autophagy and in immune signaling, participating to the control of metabolic homeostasis.
-
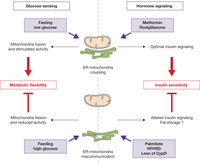
Figure 3
Role of ER–mitochondria miscommunication in metabolic diseases. At top: optimal ER–mitochondria interactions are required for metabolic flexibility (left) and insulin signaling (right). Top left: increased organelle interactions at fasting state promote mitochondria fusion and maximal oxidative capacities to predominantly oxidize lipids. Top right: antidiabetic drugs improve both insulin sensitivity and organelle interactions in diabetic mice. At the bottom: ER–mitochondria miscommunication is involved in metabolic inflexibility during nutritional transition (left) and in insulin resistance (right). Bottom left: high glucose levels reduce ER–mitochondria interactions at post-prandial state, presumably leading to the storage of excess of glucose into lipids. Bottom right: palmitate treatment, high-fat and high-sucrose diet (HFHSD) feeding or loss of cyclophilin D (CypD) reduces both organelle interactions and insulin sensitivity.
-
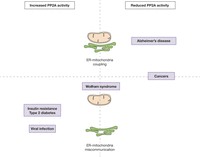
Figure 4
Hypothetical relationship between PP2A activity and ER–mitochondria interactions in several metabolic diseases. Several diseases associated with disrupted metabolism, such as type 2 diabetes mellitus (T2DM), Alzheimer’s diseases or viral infection show deregulated PP2A activity (upregulated at left or downregulated at right) and change in ER–mitochondria contact (reinforcement at top and disruption at bottom). Wolfram syndrome shows ER–mitochondria miscommunication, but no link with PP2A has been made. At opposite, cancers are associated with reduced PP2A activity, but MAM integrity is unknown.
- © 2017 Society for Endocrinology