FOXL2: a central transcription factor of the ovary
- Adrien Georges1,2,
- Aurelie Auguste1,2,
- Laurianne Bessière1,2,
- Anne Vanet1,2,
- Anne-Laure Todeschini1,2 and
- Reiner A Veitia1,2
- 1CNRS UMR 7592, Institut Jacques Monod, 15 Rue Hélène Brion, 75013 Paris, France
2Université Paris Diderot, Paris VII, Paris, France
- Correspondence should be addressed to R A Veitia; Email: veitia.reiner{at}ijm.univ-paris-diderot.fr
-
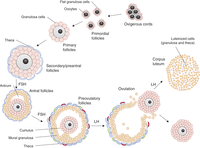
Figure 1
Outline of the main steps of folliculogenesis. Folliculogenesis starts with primordial follicles that are subsequently recruited to become primary follicles. Proliferating GCs progressively form several layers around the oocyte (secondary to late preantral follicles). From this stage, a layer of theca cells surrounds the follicle, and the follicles start to produce oestrogens. Theca cells produce androgens, which are converted into oestrogens in granulosa cells. Whereas follicular growth to the secondary/preantral stage is independent of gonadotrophins, progression beyond this stage strictly depends on FSH stimulation. As GC continue to proliferate, the antrum is formed and separates de facto granulosa cells into two general populations: cumulus and mural GCs. At this stage, selection occurs between growing follicles, and only one or a limited number of follicles continue growing to the preovulatory stage while others undergo atresia. Two layers of theca (theca interna and externa) can be distinguished around selected follicles. After ovulation, which is triggered by a peak of FSH and LH, theca cells and mural granulosa cells luteinise to produce progesterone.
-
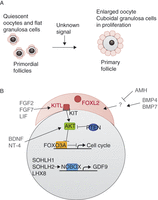
Figure 2
Overview of some factors implicated in follicle recruitment for maturation. (A) Summary and representation of principal macroscopic changes between primordial and primary follicles. (B) Representation of the principal factors implied to be involved in primordial follicle activation, and their relations, when they are known. Ooocyte-transcribed factors are in black, granulosa-transcribed factors are in red and factors originating from other follicles or of unknown origin are in grey. Activation is represented by an arrow and repression by a blunt arrow.
-
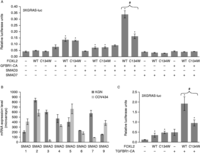
Figure 3
Mutant FOXL2 fails to cooperate with SMAD3 on a composite regulatory element. (A) Luciferase activity of the reporter promoter 3XGRAS-luc in COV434 cells. Reporter vector (400 ng) was co-transfected with 200 ng of each of FOXL2 WT or C134W, TGFBR1-CA, SMAD3 and SMAD7, or pcDNA3.1 control vector, as indicated. Error bars represent the s.d. of five replicates. Asterisks represent a Student's t-test P value <10−3 between the indicated condition and control condition. The # symbol indicates a Student's t-test P value <10−3 between the FOXL2 WT and mutant conditions. (B) Expression value of SMAD1–9 mRNAs in COV434 cells and KGN cells, determined from microarray data (GEO datasets GSE39890 from Rosario et al. (2012)). Error bars represent the s.d.s of three conditions. (C) Luciferase activity of the reporter promoter 3XGRAS-luc in KGN cells. Reporter vector (400 ng) was co-transfected with 300 ng of each of FOXL2 WT or C134W, TGFBR1-CA or pcDNA3.1 control vector, as indicated. Error bars represent the s.d. of five replicates. Asterisks represent a Student's t-test P value <10−3, between the indicated condition and control condition. The # symbol indicates a Student's t-test P value <10−3 between the FOXL2 WT and mutant conditions. Experiments were led as in L'Hôte et al. (2012) and Benayoun et al. (2013).
- © 2014 Society for Endocrinology