- Made available online as an Accepted Preprint 16 February 2009
- Accepted Preprint first posted online on 16 February 2009
Cortisol mobilizes mineral stores from vertebral skeleton in the European eel: an ancestral origin for glucocorticoid-induced osteoporosis?
- Miskal Sbaihi1,
- Karine Rousseau1,
- Sylvie Baloche1,
- François Meunier1,
- Martine Fouchereau-Peron1,2 and
- Sylvie Dufour1
- 1Muséum National d'Histoire Naturelle, DMPA USM 0401, UMR 7208 CNRS BOREA ‘Biologie des Organismes et Ecosystèmes Aquatiques’, 7 rue Cuvier, CP 32, 75231 Paris Cedex 05, France2Marine Station of Concarneau, 29182 Concarneau Cedex, France
- (Correspondence should be addressed to S Dufour; Email: dufour{at}mnhn.fr)
Abstract
Endogenous excess cortisol and glucocorticoid (GC) therapy are a major cause of secondary osteoporosis in humans. Intense bone resorption can also be observed in other vertebrates such as migratory teleost fish at the time of reproductive migration and during fasting when large amounts of calcium and phosphate are required. Using a primitive teleost, the European eel, as a model, we investigated whether cortisol could play an ancestral role in the induction of vertebral skeleton demineralization. Different histological and histomorphometric methods were performed on vertebral samples of control and cortisol-treated eels. We demonstrated that cortisol induced a significant bone demineralization of eel vertebrae, as shown by significant decreases of the mineral ratio measured by incineration, and the degree of mineralization measured by quantitative microradiography of vertebral sections. Histology and image analysis of ultrathin microradiographs showed the induction by cortisol of different mechanisms of bone resorption, including periosteocytic osteolysis and osteoclastic resorption. Specificity of cortisol action was investigated by comparison with the effects of sex steroids. Whereas, testosterone had no effect, estradiol induced vertebral skeleton demineralization, an effect related to the stimulated synthesis of vitellogenin (Vg), an oviparous specific phospho-calcio-lipoprotein. By contrast, the cortisol demineralization effect was not related to any stimulation of Vg. This study demonstrates GC-induced bone demineralization in an adult non-mammalian vertebrate, which undergoes natural bone resorption during its life cycle. Our data suggest that the stimulatory action of cortisol on bone loss may represent an ancestral and conserved endocrine regulation in vertebrates.
Introduction
Osteoporosis is a major disease affecting human bone. It is commonly divided into primary osteoporosis, which is an inheritable metabolic bone disease, and secondary osteoporosis, which is caused by endocrinological disorders and drugs.
Glucocorticoid-induced osteoporosis (GIO), in pathological conditions of endogenous hypercortisolism such as Cushing's syndrome (Mancini et al. 2004) or after cortico-therapy, has been well demonstrated in human bone (Mazziotti et al. 2006, Canalis et al. 2007). GIO is the most common form of secondary osteoporosis and represents increasing concern for human health and therapy (Tamura et al. 2004, Mazziotti et al. 2006, Canalis et al. 2007). Chronic treatments with synthetic glucocorticoids (GC) are commonly used in autoimmune, pulmonary, and gastrointestinal diseases (Mazziotti et al. 2006). Many studies have been performed to seek a suitable animal model that could mimic bone disorder observed in patients with GIO. In vivo models have provided many data on the deleterious effects of GC excess on bone (rat: Lindgren et al. 1983, Jowell et al. 1987, Goulding & Gold 1988; mouse: Altman et al. 1992, Weinstein et al. 1998, 2002; ewes: Chavassieux et al. 1997; dogs: Quarles 1992). However, in vitro studies in rodents have been contradictory with either stimulation (rat: Gronowicz et al. 1990; mouse: Reid et al. 1986, Conaway et al. 1996, Weinstein et al. 2002) or inhibition (rat: Stern 1969, Raisz et al. 1972, Tobias & Chambers 1989, Dempster et al. 1997) of bone resorption.
Intense bone resorption can also be observed in non-mammalian vertebrates such as migratory teleost fish during sexual maturation. This was shown in naturally matured conger eels (Conger conger: Lopez & Deville-Peignoux 1974) and salmons (Salmo salar: Kacem et al. 2000, Kacem & Meunier 2003), as well as in experimentally matured European eels (Anguilla anguilla: Lopez & Martelly-Bagot 1971, Lopez 1973) and Japanese eels (Anguilla japonica: Yamada et al. 2002). It is well known that sexual maturation is characterized in fish as in other vertebrates, by high plasma levels of sex steroids. However, other hormones including cortisol may also show large variations during sexual maturation. Studies in salmon (Sower & Schreck 1982, Martin et al. 1986, Carruth et al. 2000a) showed that very high plasma levels of cortisol are observed during the final phase of reproduction in males as well as in females, pointing out the occurrence of a syndrome similar to that of Cushing's.
In fish, as in other vertebrates, cortisol is known as a major hormone of the intermediary metabolism, as well as the principal mediator of the response to stress. Its role in the mobilization of energy and metabolite reserves, in particular during periods of fasting and reproduction, was demonstrated in teleosts (for review: Mommsen et al. 1999). By contrast, its potential role in the mobilization of mineral reserves has received so far little attention. In teleosts, cortisol is in addition a key actor in osmoregulation, being involved in both ion uptake and secretion (for reviews: Boeuf & Payan 2001, McCormick 2001). In eels, cortisol may act on the gills of freshwater fish to aid in the absorption of sodium and chloride (European eel: Mayer et al. 1967, American eel: Perry et al. 1992), while it is a sodium excreting factor in fish adapted to seawater (Mayer et al. 1967).
It is known that the need for calcium and phosphate is strongly increased during sexual maturation and migration in fish (Persson et al. 1997, 1998, Kacem et al. 2000, Yamada et al. 2002). Calcium and phosphate are usually taken from the external environment (food and water), but if the availability in the external medium is insufficient, they can be drawn from the mineral-bearing elements i.e. scales and bone (A. anguilla: Lopez & Martelly-Bagot 1971; Carassius auratus: Mugiya & Watabe 1977; Oncorhynchus mykiss: Carragher & Sumpter 1991). In migratory fish, the time of reproductive migration synchronizes with a period of starvation, and consequently, the organic matter as well as minerals (calcium and phosphate in particular) is mobilized from the internal medium. Eels have a complex and particular life cycle. During juvenile growth that occurs during the freshwater phase of their life cycle (called yellow stage), eels grow and accumulate metabolic and mineral reserves. At the end of the yellow phase, they metamorphose into silver eels (Aroua et al. 2005), stop feeding and growing, and initiate their long oceanic reproductive migration during which they will mobilize their reserves. In female European eels, the concentrations of both estradiol (E2) and testosterone increase between yellow and silver stages (Sbaihi et al. 2001, Aroua et al. 2005) and rise further during experimental maturation (Leloup-Hatey et al. 1988, Peyon et al. 1997). Van Ginneken et al. (2007) reported that plasma cortisol levels in European eels also increased between yellow and silver stages.
Considering that scales in eel are poorly developed compared with other teleosts (Zylberberg et al. 1984), this species provides a relevant model for investigating the role of the internal skeleton, and in particular the long vertebral skeleton, in the storage and mobilization of minerals. Yamada et al. (2002) found that during experimental maturation in female Japanese eels, the calcium content of the skin (which contains the scales) did not decrease, while the vertebral skeleton constituted the principal source for calcium and phosphate. Furthermore, the eel has a cellular bone (containing osteocytes in its matrix), in contrast to most teleosts which present acellular bone, but similar to other vertebrates (Lopez 1970a, Francillon-Vieillot et al. 1990, Meunier & Huysseune 1992, Sire & Huysseune 2003), represents a good experimental model to study the different types of bone resorption. Finally, as a representative of a vertebrate group of ancient origin (Elopomorphes), this species could also provide important information on ancestral regulation of bone resorption.
In our study, we investigated whether cortisol could play a major physiological role in bone remodeling in a non-mammalian vertebrate. We undertook histological and histophysical (qualitative and quantitative microradiographs) studies on vertebral bone of control and cortisol-treated silver eels, according to the methods we recently developed in the eel (Sbaihi et al. 2007). We also investigated the specificity of cortisol action by comparing its effects with those of sex steroids.
Materials and Methods
Animals
Two batches of female European silver eels (A. anguilla) were caught from ponds in the north of France (Somme, France) by professional fishermen at the time of their downstream migration (mid-November) during two consecutive years. At the silver stage, eels have ended the juvenile growth phase and are initiating their reproductive migration towards the ocean. The animals were immediately transferred to the laboratory (MNHN, Paris, France) and kept in running aerated freshwater (15±2 °C). As eels undergo a natural starvation period at the silver stage, they were not fed during experiments. Animal manipulations were performed under the supervision of authorized investigators.
Hormonal treatment
Two weeks after their transfer at Muséum National d' Histoire Naturelle (December), eels were divided into experimental groups in separate tanks (15±2 °C; 4 eels/100-L tank; 8 eels=2 tanks/treatment) and hormonal treatments were started (weekly i.p. injections of steroid hormones or saline over a 3 month period). Two independent experiments were done on independent eel batches for two consecutive years.
Experiment 1
Sixteen eels (mean body weight (BW) 385±9 g) were divided into two groups: eight control eels and eight eels treated with cortisol (F; Sigma-Aldrich Corp.). Animals received a chronic treatment with cortisol (one i.p. injection per week of 2 μg steroid suspended in 0.9% NaCl/g BW), according to the protocol by Huang et al. (1999). The control group was injected with saline alone (0.9% NaCl).
Experiment 2
In order to compare the effect of cortisol with those of sex steroids, 32 eels (mean BW 400±12 g) were divided into four groups: eight control eels, eight eels treated with cortisol (F), eight eels treated with testosterone (Sigma) and eight eels treated with E2 (Sigma). As in Experiment 1, animals received a chronic treatment with steroids (one i.p. injection per week of 2 μg steroid suspended in 0.9% NaCl/g BW) according to the protocol by Huang et al. (1999) and Weltzien et al. (2006). The control group received saline alone.
At the end of both experiments, fish were killed by decapitation 1 week after the last injection. Blood was collected on heparin and plasma, obtained after centrifugation, was stored at −20 °C until steroid, calcium, phosphate, and vitellogenin (Vg) assays. For each animal, a portion of vertebral skeleton was sampled 2 cm behind the anal region according to Sbaihi et al. (2007).
Immunoassays of steroids
Plasma levels of steroids were measured at the end of Experiment 2 using ELISA (AbCys S.A., Paris; E2: DNOV003; testosterone: DNOV002; cortisol: DNOV001). At the end of the 3-month treatment, plasma testosterone levels were higher in testosterone-treated eels (17.02±5 ng/ml) than in controls (2.5±0.5 ng/ml). Plasma E2 levels were also higher in E2-treated eels (80.57±9.3 ng/ml) than in controls (4.2±0.8 ng/ml). These increases were similar to those previously observed (Weltzien et al. 2006, Aroua et al. 2007). By contrast, plasma cortisol levels at the time of killing did not differ between treated and control eels (32.74±7.47 ng/ml in cortisol-treated eels versus 34.08±7.80 ng/ml in controls). In order to further investigate the impact of cortisol injections, a kinetic study of plasma cortisol levels after i.p. injection of cortisol (2 μg/g BW) or of saline was performed (four eels/group). Blood samples were taken from the caudal vasculature at 4, 8, 24, 48 h, 4, and 7 days after injection. Throughout the kinetic study, plasma cortisol levels remained between 20 and 150 ng/ml in control eels. Eight hours after injection, plasma cortisol levels peaked at 1000–1500 ng/ml in cortisol-injected eels, decreased to 300–500 ng/ml at 24 h, and returned to basal levels at 48 h, as well as at 4 and 7 days. Previous studies showed that the metabolic clearance rate for cortisol in European eel was 1500 to 2500 ml/kg BW/day (Leloup-Hatey 1976), a value much higher than those for testosterone (41 ml/kg BW/day; Quérat et al. 1985) and E2 (12 ml/kg BW/day; Quérat et al. 1985). These clearance rates are in agreement with the respective plasma levels observed 7 days after the last injection in the present study. It was also reported that the secretion rate for cortisol in European eel was 210 to 560 ng/g BW/7 days (Leloup-Hatey 1976), which means that administration of 2 μg/g BW per week corresponds to 3–10 times the basal production of cortisol.
Calcium and phosphate assays
Plasma levels of calcium and phosphate were measured spectrophotometrically at the end of Experiment 2, using the Ca-Kit 61041 and PHOS UV 61571 respectively (bioMérieux, Craponne, France). Each sample was measured in duplicate and data expressed as mg/dl.
Immunoassay of vitellogenin
Vg was assayed in plasma samples at the end of Experiment 2, using a homologous ELISA for European eel Vg (Sbaihi et al. 2001).
Histology of vertebrae
Vertebrae were fixed in 70% alcohol and stained using basic fuchsine at 1% (Frost 1959). They were dehydrated in a graded series of ethanol and in xylene, and subsequently embedded in prepolymerized methacrylate (Reinhold 1997). Slides were cut into 30±5 μm and mounted in Canada balm. Observations were made under a light microscope (LEICA MZ APO).
Measurement of mineral ratio by incineration
Vertebral samples were cleaned, dried, and weighed (dry weight) to a precision of 0.01 mg (Mettler AC 100), incinerated for 7 hours at 850 °C and the ashes weighed (mineral weight) to a precision of 0.01 mg, as described earlier (Sbaihi et al. 2007). The mineral ratio (MR) which is the quantity of mineral per unit mass of dried material was calculated as followed: MR (%)=(mineral weight/dry weight)×100.
Measurement of mineralization degree by quantitative microradiography
This method has been previously developed (Sissons et al. 1960, Boivin & Baud 1984) and recently used in studies on human bone biopsies (Boivin et al. 2000) and fish vertebrae (Salmo salar: Kacem & Meunier 2003; European eel: Sbaihi et al. 2007). Briefly, slices (100±1 μm) of embedded (Stratyl: chronolite 2195) vertebrae were microradiographied with a CGR Sigma 2060 X-ray generator. The settings of the X-ray unit were 20 kV, 7 mA, 60 min exposure time and a distance of 40 cm between the beam source and the X-ray film (Sbaihi et al. 2007). The microradiographied vertebrae were compared with the optical density of 99.9% pure foil aluminium standard (Strems Chemical Ltd, Strasbourg, France) of increasing thickness (Boivin & Baud 1984, Meunier & Boivin 1997, Kacem & Meunier 2003, Sbaihi et al. 2007). Data could be converted from gray-level values (optical densities) to degree of mineralization, i.e. the quantity of mineral substance present in a unit of bone volume, and expressed as equivalent of g mineral/cm3 bone (Sissons et al. 1960, Boivin et al. 2000, Kacem & Meunier 2003, Sbaihi et al. 2007). On each microradiograph, surface areas 100±1 μm thickness were digitalized, selected surface areas were edited and analyzed by NIH-Image 1.61 program. Eight zones in the mineralized regions were measured in each section, and the average was considered as the value of mineralization degree (MD) for the corresponding vertebra.
Measurement of the periosteocytic lacunae surface area
Ultrathin slices (<20 μm) of embedded vertebrae were prepared and microradiographied with a CGR Sigma 2060 X-ray without nickel filter. The settings of the X-ray unit were 10 kV, 5 mA, 5 min exposure time, and a distance of 5 cm between the beam source and the X-ray film (Sbaihi et al. 2007). Microradiographs were digitalized and selected surface areas edited and analyzed by NIH-Image 1.61 program. The surface area of 40 osteocytic lacunae was measured on each slide and the average was considered as the value of lacunae surface area for the corresponding vertebra. The results were expressed in μm2.
Statistical analysis
Data from control and steroid-treated eels were expressed as mean±s.e.m. Data were analyzed by Student's t-test or one-way ANOVA followed by Student-Newman-Keuls multiple comparison tests, using InStat (GraphPad Software). Differences were considered significant at P<0.05.
Results
Vertebral histological structure and effect of cortisol on osteoclastic resorption
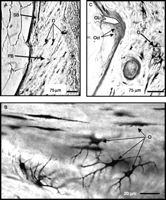
The microscopic examination of the vertebrae sections of control eels showed primary bone (primary mineralized tissue) and at the periphery of this one, zones of secondary bone (new mineralized bone), which differed by the orientation of the bone structure (Fig. 1A). In all sections, the osteocytes were easily identifiable and strongly stained with fuchsine. The existence of osteocytes inside the bone structure means that eel bone belongs to the cellular type (osteocytic bone), as first mentioned by Lopez (1973). High magnification showed that osteocytes present a spangled form (the shape of spider; Fig. 1B).
Histology of eel vertebral bone. Vertebrae were stained with basic fuchsine, embedded in methacrylate and cut at 30±5 μm (A) Vertebral section from a control eel showing primary bone (PB) and secondary bone (SB) indicating bone remodeling. Many osteocytes (O) are included in the bone structure demonstrating a cellular (osteocytic) bone (Scale bar=75 μm). (B) Higher magnification of a vertebral section from a control eel showing the spangled form of the osteocytes (O; Scale bar=20 μm). (C) Vertebral section of a cortisol-treated eel showing a large osteoclastic lacuna (OL) and the presence of osteoclasts (Ocl; Scale bar=75 μm).
Histological study revealed the presence of large cavities of resorption (Howship's lacunae) at the periphery of the vertebral bone in cortisol-treated eels (Fig. 1C). These lacunae were strongly stained by fuchsine in contrast to the non-resorbed bone. Pluri-nucleated osteoclasts, known to be responsible for osteoclastic resorption in higher vertebrates, could be observed in these lacunae. Such large lacunae were not observed in vertebral sections of control eels. These results suggested that chronic treatment with cortisol stimulated osteoclastic resorption.
Effect of cortisol on MR
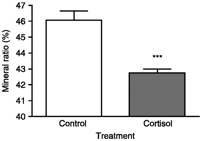
Measurement of MR% by the method of incineration showed a significant (P<0.001) decrease in the mineral content of vertebrae from cortisol-treated eels (42.76±0.23%) as compared with controls (46.07±0.56%; Fig. 2). This indicated an important mineral loss after treatment with cortisol.
Effect of cortisol on MD
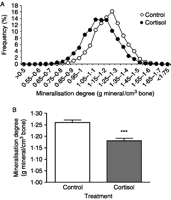
The measurement of the MD, which represents the quantity of mineral per unit of bone volume, was carried out by image analysis of the quantitative microradiographs (sections 100±1 μm). The distribution frequency of the MD measurements made on eight zones of each microradiograph of 100±1 μm vertebral section is shown in Fig. 3A. The shift of the peak of MD in eels treated with cortisol compared with control eels indicated a global reduction of the MD in treated eels. A significant (P<0.001) decrease in mean values of MD was observed between vertebrae from cortisol-treated eels (1.18±0.01 g/cm3) and vertebrae from control eels (1.26±0.01 g/cm3; Fig. 3B).
Effect of cortisol on mineralization degree (MD) of eel vertebrae. MD (g mineral/cm3 of bone) is measured by quantitative microradiography of vertebral sections (100±1 μm) of control and cortisol-treated eels. (A) Distribution of MD values in vertebrae from control and cortisol-treated eels (eight vertebral zones measured per eel vertebral section; eight control eels and eight treated eels). (B) Mean MD±s.e.m. of vertebrae from control and cortisol-treated eels (n=8 eels/group). ***P<0.001 as compared with controls.
Effect of cortisol on periosteocytic osteolysis
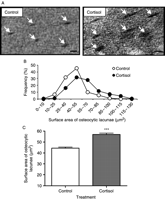
Microradiographs of ultrathin vertebral sections (20 μm; Fig. 4A) revealed that the osteocytic lacunae observed in cortisol-treated eels were larger than those in control eels. The distribution frequency of the lacunae surface area measured on forty osteocytic lacunae for each microradiograph of <20 μm vertebral section is shown in Fig. 4B. The shift of the peak in eels treated with cortisol compared with control eels indicated a global increase of the lacunae surface area in treated eels. This indicated an activation of the periosteocytic osteolysis in cortisol-treated eels. The mean surface area of osteocytic lacunae from vertebra sections of control and cortisol-treated eels is shown in Fig. 4C. In cortisol-treated eels, the average surface area of osteocytic lacunae was significantly increased as compared with controls (56.93 μm2±1.2 vs 44.47±0.8 μm2 P<0.001; Fig. 4C).
Effect of cortisol on periosteocytic resorption of eel vertebrae. (A) Microradiographs of ultrathin vertebral sections (<20 μm) from control and cortisol-treated eels, showing periosteocytic lacunae (arrows). The surface areas of lacunae are measured by image analysis of microradiographies. Osteocytic lacunae are larger in cortisol-treated eels than in controls. (Scale bar=10 μm). (B) Distribution of surface area of periosteocytic lacunae in vertebral sections of control and cortisol-treated eels (40 osteocytic lacunae measured/eel; eight control eels and eight treated eels). (C) Mean surface area of periosteocytic lacunae in vertebrae from control and cortisol-treated eels. Results are expressed as mean±s.e.m. (n=8 eels/group). ***P<0.001 as compared with controls.
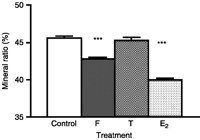
Comparison of the effects of cortisol and sex steroids on MR
In agreement with Experiment 1 (Fig. 2), vertebral mineral content was significantly decreased (P<0.01) by cortisol treatment (42.54±0.32%) as compared with controls (45.59±0.22%) in Experiment 2 (Fig. 5). A significant decrease in MR was also observed in E2-treated eels (39.93±0.22%, P<0.001; Fig. 5). By contrast, no significant change was observed with testosterone (45.26±0.40%; Fig. 5).
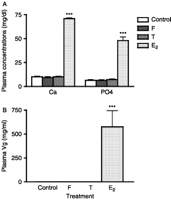
Comparison of the effects of cortisol and sex steroids on calcium, phosphate, and Vg plasma concentrations
Plasma calcium concentrations (Fig. 6A) were unchanged in cortisol- and testosterone-treated eels (9.29±1 mg/dl and 10.16±0.45 mg/dl respectively) as compared with controls (10.20±0.4 mg/dl). By contrast, E2 treatment induced a large and significant increase in calcium plasma levels (70.9±0.7 mg/dl; P<0.001; Fig. 6A).
Comparison of the effects of cortisol and sex steroids on plasma concentrations of calcium, phosphate (A) and vitellogenin (B) in the European eel. Plasma concentrations of calcium and phosphate are measured spectrophotometrically and vitellogenin by homologous ELISA. Results are expressed as mean±s.e.m. (n=8 eels/group). ***P<0.001 as compared with controls.
Similarly, plasma phosphate concentrations (Fig. 6A) were unchanged in cortisol- and testosterone-treated eels (6.42±0.66 mg/dl and 7.17±0.58 mg/dl respectively) as compared with control eels (6.31±0.64 mg/dl), while E2 treatment induced a large and significant increase in phosphate plasma levels (47.93±3.72 mg/dl; P<0.001; Fig. 6A).
A drastic increase (more than ×105) of plasma Vg concentrations was observed after E2 treatment (576±170 mg/ml vs <2 μg/ml for controls; P<0.001). By contrast, plasma Vg concentrations remained low (<2 μg/ml) in testosterone- and cortisol-treated eels (Fig. 6B).
Discussion
The strong vertebral bone demineralization, observed in the present study, after chronic cortisol treatment in a primitive teleost suggests that GC induction of bone loss may represent an ancestral regulatory mechanism, conserved during vertebrate evolution.
Cortisol-induced eel vertebral demineralization
In the European eel, chronic treatment with cortisol induced a significant demineralization of the vertebral skeleton, as shown by the reduction of MR measured by incineration. This method has already been used in rat (Wink & Felts 1980, Ortoft & Oxlund 1988) and fish (Casadevall et al. 1990, Kacem et al. 2000), for studying bone demineralization. In the Japanese eel, Yamada et al. (2002) demonstrated by this method that the mineral content (phosphorus and calcium) in bone tissue decreased during experimental sexual maturation. In the European eel, we previously showed by this method the effect of thyroid hormone on vertebral demineralization (Sbaihi et al. 2007).
The demineralization of eel vertebrae after cortisol treatment was further demonstrated by measuring the MD, using quantitative microradiographies. A reduction of MD has been previously shown in eel vertebra during experimental maturation (Lopez et al. 1970, Lopez & Martelly-Bagot 1971) or after chronic treatment with thyroid hormones (Sbaihi et al. 2007), as well as in Atlantic salmon during the anadromous reproductive migration (Kacem & Meunier 2003). Our data suggest that cortisol, the levels of which increase during sexual maturation in fish (Donaldson & Fagerlund 1972, Pickering & Christie 1981, Sower & Schreck 1982, Ueda et al. 1984), may be involved physiologically in the maturation-related bone demineralization. Measurement of MD has also been previously used in mammals in experimental osteoporosis studies (Jowsey et al. 1965, Baud et al. 1980, Meunier & Boivin 1997) and in post-menopausal osteoporotic women on transiliac bone biopsies (Boivin et al. 2000).
Recently, zebrafish larva was proposed as a rapid in vivo model of GIO (Barrett et al. 2006) using staining mineralized tissue with fluorescent dye as a method to quantify bone size and density (Du et al. 2001). The authors exposed zebrafish larvae in 96-well plates to different doses of GC pharmacological agonist (prednisolone) for 5 days. The data revealed a marked bone loss in larvae treated with prednisolone. Furthermore, this prednisolone-induced bone loss was prevented by RU486, a glucocorticoid receptor (GR) antagonist (Barrett et al. 2006). Our present study demonstrates that GIO also occurs in the adult European eel. These data suggest that the bone demineralization effect of GCs may represent a general regulatory process in teleosts, since it may occur at different life stages (larvae or adults) and in species representative of different teleost groups (cypriniformes for zebrafish and elopomorphs for eel). Cortisol mobilization of skeletal mineral stores likely reflects a physiological process especially in the case of long migratory and fasting fish, such as eels and salmons. In addition, this regulatory pathway could lead to stress-induced pathological impairment of skeleton development and homeostasis in fish. For instance, further investigations could decipher whether GIO may participate in skeletal abnormalities largely observed in aquaculture (Deschamps et al. 2008).
Cortisol-induced osteocytic osteolysis
The histological observation of eel vertebral bone showed the presence of numerous osteocytes inside the bone structure, demonstrating that eel vertebra, as in higher vertebrates but different from the majority of other teleosts, is a cellular bone (osteocytic bone; Weiss & Watabe 1979, Meunier & Huysseune 1992, Eckhard-Witten 1997). The eel thus provides an interesting comparative model for the study of the osteocytic mechanisms of bone resorption. We showed in the present study, using image analysis of ultrathin microradiographs, that treatment with cortisol induced a significant increase in the surface area of the osteocytic lacunae. This indicates a stimulation of osteocytic osteolysis by cortisol in eel vertebral bone.
In fish, osteocytic resorption was previously shown to increase in the eel during experimental maturation (Lopez 1970b, Lopez & Martelly-Bagot 1971) and after chronic treatment with thyroid hormones (Sbaihi et al. 2007), and in the salmon during natural maturation (Baud 1962, Kacem & Meunier 2000). Periosteocytic osteolysis resorption has also been observed in the vertebrae of a snake, Vipera aspis, during reproduction (Alcobendas & Baud 1988, Alcobendas et al. 1991).
Numerous studies in mammals have demonstrated that osteocytes are highly active cells and that mature osteocytes are capable of resorbing mineral substance as well as organic matrix from their surrounding bone under physiological and pathological conditions (for reviews: Kogianni & Noble 2007). In humans, osteoporosis caused by long-term GC treatment is accompanied by a significant enlargement of the periosteocytic lacunar surface area (Baud & Boivin 1978, Wright et al. 1978). In GC-treated mice, larger osteocyte lacunae were also observed on vertebral bone sections (Lane et al. 2006).
The present study is the first demonstration of a stimulatory effect of cortisol on osteocytic osteolysis in a non-mammalian vertebrate. Our data suggest that cortisol-induced osteocytic osteolysis may represent an ancient and conserved cellular mechanism of bone resorption in vertebrates.
It is not yet known whether the perilacunar resorption is due to a direct action of GC on the eel bone cells. The presence of GR on osteocytes was demonstrated in rat (Silvestrini et al. 1999) and in human (Abu et al. 2000). This suggests that the action of GC on osteocyte activity may be directly mediated via the GR. In fish, two distinct functional GR were cloned in rainbow trout (Ducouret et al. 1995, Bury et al. 2003). GR have been demonstrated in a variety of salmonid tissues (Maule & Schreck 1991, Maule et al. 1993, Knoebl et al. 1996, Carruth et al. 2000b, Bury et al. 2003), but until now, there is no information on the presence of GR in the bone tissue in fish. Further investigation in the eel could address this question.
Cortisol-induced osteoclastic resorption
The microscopic observation showed large lacunae of osteoclastic resorption at the periphery of the vertebral bone of cortisol-treated eels. This type of resorption has already been described in the female European eel under experimental maturation and in the female conger during spontaneous maturation (Lopez 1970b, Lopez & Martelly-Bagot 1971).
In mammals, GC treatments have a wide spectrum of effects on osteoclastic resorption according to models and studies. In humans, a biphasic effect of GC on osteoclasts has been hypothesized: GC could have an acute inhibitory effect on osteoclast synthesis without significant modification of bone resorption; on the contrary, in the long-term, the GC-mediated stimulation of osteoclast synthesis might be coupled with an increase in bone resorption (for review: Manelli & Giustina 2000). In rodents, GC was shown to decrease basal activity of osteoclasts (Wong 1979), to impair osteoclastogenesis (Weinstein et al. 1998) and to reduce the number of osteoclasts (in vivo: Lindgren et al. 1983, Jowell et al. 1987; in vitro: Tobias & Chambers 1989, Dempster et al. 1997). By contrast, other authors indicated a stimulatory effect of GC on osteoclastic resorption in rodents. Some studies have suggested a possible direct effect of GC on osteoclast-mediated bone resorption, as stimulation of bone osteoclastic resorption by GC was observed in organ cultures of fetal rat parietal bones and neonatal mouse calvariae (Gronowicz et al. 1990, Conaway et al. 1996). Furthermore, GC has been shown to stimulate osteoclast formation in bone marrow cultures in the mouse (Shuto et al. 1994) and to extend the life span of pre-existing osteoclasts in murine osteoclast cultures (Weinstein et al. 2002). In another model, the dog, as in the mouse, a significant decrease of trabecular bone volume was observed in vivo after treatment with GC (Altman et al. 1992, Quarles 1992, Weinstein et al. 1998, McLaughlin et al. 2002). Our present study indicates that GC-induced osteoclastic resorption would also occur in a non-mammalian vertebrate, in which it may represent a physiological mechanism of mobilization of mineral stores.
E2 action on bone demineralization: an oviparous feature
To investigate the specificity of cortisol action, we compared its effects with those of sex steroids. Our data showed that, like cortisol, E2 induced a decrease of vertebral MR. However, in contrast to cortisol, E2 also induced a drastic increase in plasma Vg concentrations, as well as in plasma calcium and phosphate concentrations. The increase in calcium and phosphate plasma concentrations under E2 treatment reflects their involvement in the composition of Vg, which is a phospho-calcio-lipoprotein (for review: Polzonetti-Magni et al. 2004). In the eel, the large increase in plasma Vg concentrations after E2 treatment results from both the stimulation of Vg liver production and the very low Vg uptake by oocytes at the early silver stage (Burzawa-Gérard & Dumas-Vidal 1991). The specificity of E2 on both bone demineralization and Vg induction was confirmed by the lack of effect of testosterone.
By contrast, some previous studies in other teleosts showed that E2 did not induce any bone demineralization. In rainbow trout, Armour et al. (1997) reported that following E2 treatment, calcium and phosphate contents decreased in scales while they increased in bone. E2 treatment induced calcium mobilization from scales in goldfish, killifish, and rainbow trout (Mugiya & Watabe 1977, Carragher & Sumpter 1991, Persson et al. 1994). Teleost scales are calcified tissues, which may contain up to 20% of the total body calcium in some species and serve as a functional mineral reservoir during periods of increased mineral demand, such as sexual maturation and starvation (Takagi et al. 1989, Bereiter-Hahn & Zylberberg 1993, Persson et al. 1998). However, scales in eel are poorly developed which differs from other teleosts (Zylberberg et al. 1984) and cannot serve as a mineral reservoir. Yamada et al. (2002) found that during sexual maturation in female Japanese eels, the calcium and phosphorus contents of the skin (which contains the few scales) remained unchanged, while they decreased in the vertebral skeleton, which constitutes the principal source for calcium and phosphorus. Estrogen receptors were reported in bone and scales of rainbow trout (Armour et al. 1997, Persson et al. 2000), regenerating scales of goldfish (Yoshikubo et al. 2005) and bone of sea bream (Socorro et al. 2000). This suggests a possible direct effect of E2 on bone and scale demineralization in teleosts, related to the strong demand in minerals for Vg production in oviparous vertebrates.
Cortisol action on bone demineralization: a general vertebrate feature
In contrast to the effect of E2, the demineralization effect of cortisol was not related to vitellogenesis, and can therefore be considered as a general regulatory mechanism among teleosts and other oviparous and non-oviparous vertebrates, including mammals.
By using various histological and histomorphometric methods, we showed that cortisol could act through different mechanisms of bone resorption, including osteoclastic resorption and periosteocytic osteolysis. This is the first demonstration of the role of cortisol in the induction of bone loss in an adult non-mammalian vertebrate, which undergoes natural bone demineralization during its life cycle.
The stimulatory role of cortisol on bone demineralization, observed in this study in a representative of a vertebrate group of ancient origin (Elopomorphes), the European eel, could be considered as an ancestral regulation for mobilization of mineral stores in vertebrates. Nevertheless, as opposed to most vertebrates, eels die following spawning; this may led to a divergence in vertebrate evolution, in that in species such as eel, bone demineralization could potentially occur to a greater degree (‘point of no return’) as post-spawning mortality is pre-determined.
In conclusion, we demonstrated cortisol-induced demineralization of the eel vertebral bone, a phenomenon observed in human under pathological conditions such as secondary osteoporosis after hypercortisolism or GC therapies. This suggests that the stimulatory action of GC on bone loss may reflect an ancestral endocrine regulation for mobilization of mineral stores from vertebral skeleton, a regulation that would have been conserved throughout vertebrate evolution. Non-mammalian vertebrates, such as the eel, can thus provide comparative models for experimental studies of the mechanisms of osteoporosis and bone loss.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported in part by grants from MNHN (BQR) and European Community (EELREP Q5RS-2001-01836). Dr M Sbaihi was a recipient of a post-doc fellowship from the European community (EELREP).
Acknowledgements
We would like to thank Drs J-Y Sire and J Castanet (CNRS, Universities Paris 6 and Paris 7) for facilitating access to their laboratory and the use of their equipment concerning the realization of microradiographs and image analyses.
- Received in final form 3 February 2009
- Accepted 13 February 2009
- © 2009 Society for Endocrinology