Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future
- Correspondence should be addressed to K Öberg; Email: kjell.oberg{at}medsci.uu.se
-
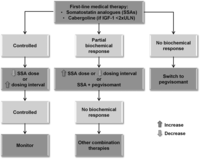
Figure 2
Algorithm for the approach to medical therapy in patients with acromegaly. Reproduced, with permission, from Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KKY, et al. (2014) Expert consensus document: a consensus on the medical treatment of acromegaly, Nature Reviews Endocrinology, volume 10, issue 4, pages 243–248. Copyright 2014, Rights Managed by Nature Publishing Group.
-
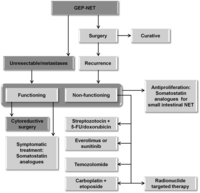
Figure 3
Algorithm for the treatment of GEP-NET. Adapted, with permission, from Oberg K, Knigge U, Kwekkeboom D & Perren A, Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Annals of Oncology, 2012, volume 23 (Supplement 7), pages vii124–vii130, by permission of Oxford University Press.
- © 2016 Society for Endocrinology