60 YEARS OF NEUROENDOCRINOLOGY: Redefining neuroendocrinology: stress, sex and cognitive and emotional regulation
-
Figure 1
The hippocampal formation is a target of adrenocortical steroids and is involved in spatial and episodic memory, as well as mood regulation. Both pyramidal neurons in Ammon's horn and neurons of the dentate express type I or mineralocorticoid (MR) and type 2 or glucocorticoid (GR) receptors.
-
Figure 2
The hippocampal formation is activated in spatial memory as in the London Cab Driver's study (Maguire et al. 1997), as well as in spatial location of food in food-caching birds and squirrels. The hippocampus is also a target of sex hormones that affect spatial memory (Sandstrom & Williams 2004). The hippocampus is also sensitive to damage by seizures, ischemia and head trauma in which glucocorticoids synergize effects of excitatory amino acid overload (Sapolsky 1990).
-
Figure 3
Four peptide/protein hormones, insulin-like growth factor 1 (IGF1), insulin, ghrelin, and leptin, are able to enter the brain and affect structural remodeling or other functions in the hippocampus. A transport process is involved, and specific receptors are expressed in hippocampus, as well as in other brain regions. Molecular sizes are indicated for each hormone in kDa: ghrelin, 3.5 kDa; leptin, 16 kDa; insulin, 5.8 kDa; IGF1, 7.6 kDa. Reproduced, with permission, from McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews 87 873–904. Copyright 2007 the American Physiological Society.
-
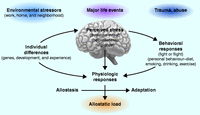
Figure 4
The stress response and development of allostatic load. The perception of stress is influenced by one's experiences, genetics, and behavior. When the brain perceives an experience as stressful, physiologic and behavioral responses are initiated, leading to allostasis and adaptation. Over time, allostatic load can accumulate, and the overexposure to mediators of neural, endocrine, and immune stress can have adverse effects on various organ systems, leading to disease. Reproduced, with permission, from McEwen BS (1998) Protective and damaging effects of stress mediators. New England Journal of Medicine 338 171–179. Copyright 1998 Massachusetts Medical Society.
-
Figure 5
The trisynaptic organization of the hippocampus showing input from the entorhinal cortex to both CA3 and dentate gyrus (DG), with feed forward and feedback connections between these two regions that promote memory formation in space and time but, at the same time, makes the CA3 vulnerable to seizure-induced excitatory (McEwen 1999). Chronic stress causes apical dendrites of CA3 neurons to debranch and shorten in a reversible manner, and glutamate release by giant mossy fiber terminals is a driving force. Chronic stress also inhibits neurogenesis in DG and can eventually reduce DG neuron number and DG volume.
-
Figure 6
Cyclic ovarian function regulates spine synapse turnover in the CA1 regions of the rat hippocampus and it does so via a combination of nuclear and non-nuclear estrogen receptors. The cell nuclear estrogen receptors are found in a subset of inhibitory interneurons whereas the non-nuclear receptors are expressed in presynaptic cholinergic and NPY terminals, in dendrites and mitochondria (McEwen & Milner 2007, Nilsen et al. 2007, Ledoux et al. 2009).
-
Figure 8
Non-genomic effects of estrogen. Estrogen initiates a complex set of signal transduction pathways in the hippocampal neuron via several membrane-bound receptors. Above are two examples of estrogen-initiated signal transduction leading to spinogenesis and changes in synapse size. Rapid activation of Akt (protein kinase B) via PI3K is thought to be mediated by ERα. Subsequently, activated Akt initiates translation of PSD-95 by removing the repression of the initiation factor 4E-binding protein1 (4E-BP1). Estradiol-mediated phosphorylation of cofilin has been shown to occur via activation of LIMK. Cofilin is an actin depolymerization factor and it is inactivated by phosphorylation. Therefore, in the presence of estrogen, cofilin repression of actin polymerization is removed, resulting in an increase in filopodial density. The signal transduction pathways illustrated here are an oversimplification of a large body of work done in an in vitro cell line. Reproduced, with permission, from Dumitriu D, Rapp PR, McEwen BS & Morrison JH (2010) Estrogen and the aging brain: an elixir for the weary cortical network. Annals of the New York Academy of Sciences 1204 104–112. Copyright 2010 New York Academy of Sciences.
-
Figure 9
Effects of stress and acute glucocorticoid treatment on gene expression in hippocampus. (A) Naïve and 21d chronically restraint stressed (CRS) rats respond differently to a 6 h bolus of corticosterone in which more than half of the genes turned on or turned off are different (Datson et al. 2013). (B) Naïve mice given acute forced swim stress (FST) show a largely different pattern of gene expression (up and down) from naïve mice given an acute corticosterone bolus. Moreover, mice that are either naïve, or 21d CRS or 21d CRS plus 21d recovery respond, in large, differently to acute FST with respect to gene expression levels. There is a core of genes that always respond to the acute FST. Reproduced, with permission, from Gray JD, Rubin TG, Hunter RG & McEwen BS 2014 Hippocampal gene expression changes underlying stress sensitization and recovery. Molecular Psychiatry 19 1171–1178. Copyright 2014, Rights Managed by Nature Publishing Group.
-
Figure 10
Novel mechanisms for rapidly acting medications to treat stress-related disorders. (A) The novel antidepressant candidate acetyl-l-carnitine (LAC) may act inside and outside the nucleus to exert fast antidepressant responses: it has been shown that LAC corrects mGlu2 deficits in vulnerable animal models by increasing acetylation of either the histone H3K27 bound to Grm2 promoter gene or the NFkB-p65 member (Nasca et al. 2013). (B) The use of the light–dark test as a screening method allows identification of clusters of animals with a different baseline susceptibility along with differences in mineralocorticoid receptor (MR) levels in hippocampus. The susceptible mice (HS, high susceptible) that are characterized by higher baseline MR levels show reduced hippocampal mGlu2 expression associated with exacerbation of anxious and of depressive-like behaviors after acute and chronic stress respectively. Conversely, individuals (LS, low susceptible) with lower baseline MR levels cope better with stress and show adaptation in mGlu2 receptor expression in hippocampus. The epigenetic allostasis model points to the developmental origins of these individual differences, suggesting that unknown epigenetic influences early in life may lead to alterations in MR hippocampal levels. Reproduced, with permission, from Nasca C, Bigio B, Zelli D, Nicoletti F & McEwen BS (2014) Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Molecular Psychiatry 20 755–763. Copyright 2014, Rights Managed by Nature Publishing Group.
- © 2015 Society for Endocrinology